Model of the nucleus of atom and the table of elements
СОДЕРЖАНИЕ: Модель атомного ядра и таблица элементовю.
Each subsequent element differs from previous that in his(its) nucleus the quantity(amount) of protons is increased by unit, and the quantity(amount) of neutrons grows, generally on some. That is in a nucleus always there are more than neutrons, than protons (not including the easiest nucleus). In the literature this strange parity(ratio) of number of neutrons to number of protons, for any nucleus, nothing speaks.
For construction of model of a nucleus of atom we shall note, that at an alpha of a radio-activity of a nucleus of helium have approximately equal to energy. Therefore on an external environment of a nucleus we shall place all protons with the same quantity(amount) of neutrons, i.e. at one power level can be only bozons with what the nucleus placed on an external environment an alpha of a particle and are. Inside a nucleus we shall arrange the staying neutrons which problem(task) will be easing electrostatic fields of pushing away of protons. Having assumed a nucleus spherical, and radiuses of a proton and a neutron approximately identical, for any element we shall receive the model of a nucleus explaining the attitude(relation) of number of neutrons to number of protons, following of packing a nucleus of atom. (Discrepancy 0-10%)
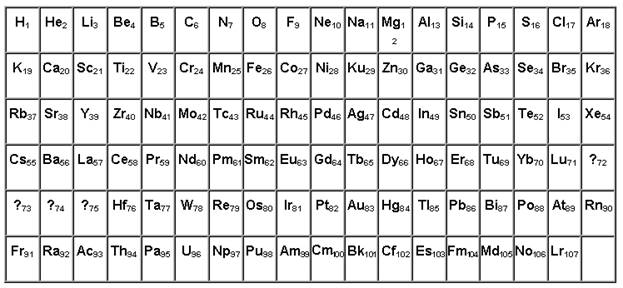
Radioactive disintegration, probably is connected to compression of a nucleus since with growth of a serial number of an element neutrons in volume of a nucleus all weaken radial forces of pushing away of protons more strongly. If weight of a nucleus to accept initial, and chemical properties of atom secondary in the table of elements atomic weight should change monotonously as across and on a vertical. Having constructed the table to these attributes we are compelled after Lu and Lr to leave on four empty places to observe chemical properties of elements.
Probably, at opening elements by necessity there is a definition of a charge of a nucleus! (the Charge of a nucleus was defined(determined) only for Cu and Pt.
 In 1891. James Chadvik has lead(carried out) experiences and with the help of formula Reserford has calculated charges of nucleus for platinum - 77,4, for silver - 46,3, for Cu-29,3. These results almost have coincided with serial numbers of these elements in table Mendeleeva.
In 1891. James Chadvik has lead(carried out) experiences and with the help of formula Reserford has calculated charges of nucleus for platinum - 77,4, for silver - 46,3, for Cu-29,3. These results almost have coincided with serial numbers of these elements in table Mendeleeva.
But, the last lanthanide are radioactive! According to our model of a nucleus of atom at the following for lanthanide elements the radio-activity could be removed(taken off) introduction inside of a nucleus of the proton environment consisting of four protons.
But then, defining(determining) a charge of a nucleus of atom of platinum on D.Chadvikas method, we again would receive value 77,4 as alpha-particles would dissipate on an external environment of a nucleus of atom. Therefore to be put a question on specification of charges of nucleus of the elements following for Hf. Can be from here and failures with hit in islet of stability .
Список литературы
Henadzi R.Filipenka ”Model of the nucleus of atom and the table of elements” Moscow “Engineer” N4 1990,N4 1991.